Blackbody Concepts
Incident energy striking an object from the surroundings, can be absorbed by the object, reflected by the object, or transmitted through the object (if it is not opaque) as seen in Figure 2-1. If the object is at a constant temperature, then the rate at which it emits energy must equal the rate at which it absorbs energy, otherwise the object would cool (emittance greater than absorption), or warm (emittance less than absorption). Therefore, for bodies at constant temperature, the emittance (absorption), the reflection and the transmittance of energy equals unity.
Central to radiation thermometry is the concept of the blackbody. In 1860, Kirchhoff defined a blackbody as a surface that neither reflects or transmits, but absorbs all incident radiation, independent of direction and wavelength. The fraction of radiation absorbed by a real body is called absorptivity, 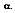 For an ideal blackbody, the absorptivity is 1.0
For an ideal blackbody, the absorptivity is 1.0  . For non-blackbodies, the absorption is a fraction of the radiation heat transfer incident on a surface, or
. For non-blackbodies, the absorption is a fraction of the radiation heat transfer incident on a surface, or  Hence, in term of radiation heat transfer, q":
Hence, in term of radiation heat transfer, q":

In addition to absorbing all incident radiation, a blackbody is a perfect radiating body. To describe the emitting capabilities of a surface in comparison to a blackbody, Kirchoff defined emissivity 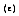 of a real surface as the ratio of the thermal radiation emitted by a surface at a given temperature to that of a blackbody at the same temperature and for the same spectral and directional conditions.
of a real surface as the ratio of the thermal radiation emitted by a surface at a given temperature to that of a blackbody at the same temperature and for the same spectral and directional conditions.
This value also must be considered by a non-contact temperature sensor when taking a temperature measurement. The total emissivity for a real surface is the ratio of the total amount of radiation emitted by a surface in comparison to a blackbody at the same temperature. The tables beginning on p. 72 give representative emissivity values for a wide range of materials. If precise temperature measurements are required, however, the surface's actual emittivity value should be obtained. (Although often used interchangeably, the terms emissivity and emittivity have technically different meanings. Emissivity refers to a property of a material, such as cast iron, whereas emittivity refers to a property of a specific surface.)
In 1879, Stefan concluded based on experimental results that the radiation emitted from the surface of an object was proportional to the fourth power of the absolute temperature of the surface. The underlying theory was later developed by Boltzmann, who showed that the radiation given off by a blackbody at absolute temperature
Ts (K) is equal to:
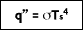
where ( is the Stefan-Boltzmann constant 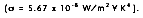 The heat transfer rate by radiation for a non-blackbody, per unit area is defined as:
The heat transfer rate by radiation for a non-blackbody, per unit area is defined as:

where Ts is the surface temperature and Tsur is the temperature of the surroundings.
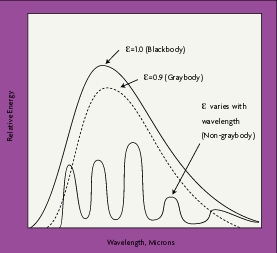 |
| Figure2-2:Spectral Distributions |
Although some surfaces come close to blackbody performance, all real objects and surfaces have emissivities less than 1. Non-blackbody objects are either graybodies, whose emissivity does vary with wavelength, or non-graybodies, whose emissivities vary with wavelength. Most organic objects are graybodies, with an emissivity between 0.90 and 0.95 (Figure 2-2).
The blackbody concept is important because it shows that radiant power depends on temperature. When using non-contact temperature sensors to measure the energy emitted from an object, depending on the nature of the surface, the emissivity must be taken into account and corrected. For example, an object with an emissivity of 0.6 is only radiating 60% of the energy of a blackbody. If it is not corrected for, the temperature will be lower than the actual temperature. For objects with an emissivity less than 0.9, the heat transfer rate of a real surface is identified as:
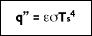
The closest approximation to a blackbody is a cavity with an interior surface at a uniform temperature Ts, which communicates with the surroundings by a small hole having a diameter small in comparison to the dimensions of the cavity (Figure 2-3). Most of the radiation entering the opening is either absorbed or reflected within the cavity (to ultimately be absorbed), while negligible radiation exits the aperture. The body approximates a perfect absorber, independent of the cavity's surface properties.
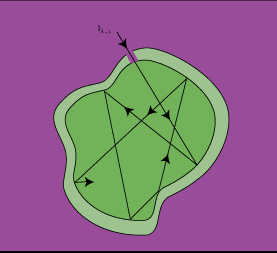 |
| Figure 2-3: An Isothermal Blacbody Cavity |
The radiation trapped within the interior of the cavity is absorbed and reflected so that the radiation within the cavity is equally distributed--some radiation is absorbed and some reflected. The incident radiant energy falling per unit time on any surface per unit area within the cavity is defined as the irradiance  If the total irradiation G (W/m2) represents the rate at which radiation is incident per unit area from all directions and at all wavelengths, it follows that:
If the total irradiation G (W/m2) represents the rate at which radiation is incident per unit area from all directions and at all wavelengths, it follows that:
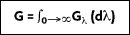
If another blackbody is brought into the cavity with an identical temperature as the interior walls of the cavity, the blackbody will maintain its current temperature. Therefore, the rate at which the energy absorbed by the inner surface of the cavity will equal the rate at which it is emitted.
In many industrial applications, transmission of radiation, such as through a layer of water or a glass plate, must be considered. For a spectral component of the irradiation, portions may be reflected, absorbed, and transmitted. It follows that:
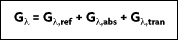
In many engineering applications, however, the medium is opaque to the incident radiation. Therefore,  and the remaining absorption and reflection can be treated as surface phenomenon. In other words, they are controlled by processes occurring within a fraction of a micrometer from the irradiated surface. It is therefore appropriate to say that the irradiation is absorbed and reflected by the surface, with the relative magnitudes of
and the remaining absorption and reflection can be treated as surface phenomenon. In other words, they are controlled by processes occurring within a fraction of a micrometer from the irradiated surface. It is therefore appropriate to say that the irradiation is absorbed and reflected by the surface, with the relative magnitudes of  depending on the wavelength and the nature of the surface.
depending on the wavelength and the nature of the surface.
Radiation transfer by a non-blackbody encompasses a wide range of wavelengths and directions. The spectral hemispherical emissive power,  is defined as the rate at which radiation is emitted per unit area at all possible wavelengths and in all possible directions from a surface, per unit wavelength
is defined as the rate at which radiation is emitted per unit area at all possible wavelengths and in all possible directions from a surface, per unit wavelength  and per unit surface area.
and per unit surface area.
Although the directional distribution of surface emission varies depends on the surface itself, many surfaces approximate diffuse emitters. That is, the intensity of emitted radiation is independent of the direction in which the energy is incident or emitted. In this case, the total, hemispherical (spectral) emissive power, 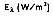 is defined as:
is defined as:
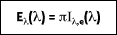
or
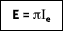
where Ie is the total intensity of the emitted radiation, or the rate at which radiant energy is emitted at a specific wavelength, per unit area of the emitting surface normal to the direction, per unit solid angle about this direction, and per unit wavelength. Notice that 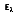 is a flux based on the actual surface area, where
is a flux based on the actual surface area, where 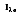 is based on the projected area. In approximating a blackbody, the radiation is almost entirely absorbed by the cavity. Any radiation that exits the cavity is due to the surface temperature only.
is based on the projected area. In approximating a blackbody, the radiation is almost entirely absorbed by the cavity. Any radiation that exits the cavity is due to the surface temperature only.
The spectral characteristics of blackbody radiation as a function of temperature and wavelength were determined by Wilhelm Wien in 1896. Wien derived his law for the distribution of energy in the emission spectrum as:
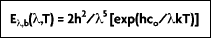
where  (b for blackbody) represents the intensity of radiation emitted by a blackbody at temperature T, and wavelength
(b for blackbody) represents the intensity of radiation emitted by a blackbody at temperature T, and wavelength 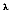 per unit wavelength interval, per unit time, per unit solid angle, per unit area. Also, h = 6.626 x 10-24 J•s and k = 1.3807 x 10-23 J•K-1 are the universal Planck and Boltzman constants, respectively; co = 2.9979 x 108 m/s is the speed of light in a vacuum, and T is the absolute temperature of the blackbody in Kelvins (K).
Due to the fact that deviations appeared between experimental results and the equation, Planck suggested in 1900 a refinement that better fit reality:
per unit wavelength interval, per unit time, per unit solid angle, per unit area. Also, h = 6.626 x 10-24 J•s and k = 1.3807 x 10-23 J•K-1 are the universal Planck and Boltzman constants, respectively; co = 2.9979 x 108 m/s is the speed of light in a vacuum, and T is the absolute temperature of the blackbody in Kelvins (K).
Due to the fact that deviations appeared between experimental results and the equation, Planck suggested in 1900 a refinement that better fit reality:
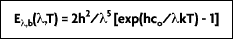
It is from this equation that Planck postulated his quantum theory. A more convenient expression for this equation, referred to as the Planck distribution law (Figure 2-4), is:

where the first and second radiation constants are 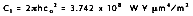 and
and 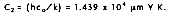 Planck's distribution shows that as wavelength varies, emitted radiation varies continuously. As temperature increases, the total amount of energy emitted increases and the peak of the curve shifts to the left, or toward the shorter wavelengths. In considering the electromagnetic spectrum, it is apparent that bodies with very high temperatures emit energy in the visible spectrum as wavelength decreases. Figure 2-4 also shows that there is more energy difference per degree at shorter wavelengths.
Planck's distribution shows that as wavelength varies, emitted radiation varies continuously. As temperature increases, the total amount of energy emitted increases and the peak of the curve shifts to the left, or toward the shorter wavelengths. In considering the electromagnetic spectrum, it is apparent that bodies with very high temperatures emit energy in the visible spectrum as wavelength decreases. Figure 2-4 also shows that there is more energy difference per degree at shorter wavelengths.
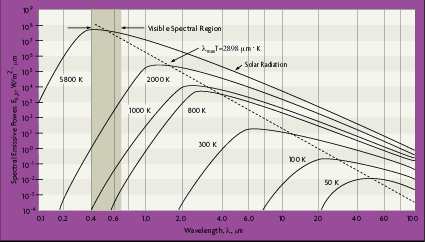 |
| Figure 2-4: Planck Prediction of Blackbody Emissive Power |
From Figure 2-4, the blackbody spectral distribution has a maximum wavelength value, lmax, which depends on the temperature. By differentiating equation 2.12 with respect to 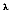 and setting the result equal to zero:
and setting the result equal to zero:
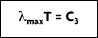
where the third radiation constant, C3 = 2897.7 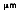 • K. This is known as Wien's displacement law. The dashed line in Figure 2-4 defines this equation and locates the maximum radiation values for each temperature, at a specific wavelength. Notice that maximum radiance is associated with higher temperatures and lower wavelengths.
• K. This is known as Wien's displacement law. The dashed line in Figure 2-4 defines this equation and locates the maximum radiation values for each temperature, at a specific wavelength. Notice that maximum radiance is associated with higher temperatures and lower wavelengths.
![]() For an ideal blackbody, the absorptivity is 1.0
For an ideal blackbody, the absorptivity is 1.0 ![]() . For non-blackbodies, the absorption is a fraction of the radiation heat transfer incident on a surface, or
. For non-blackbodies, the absorption is a fraction of the radiation heat transfer incident on a surface, or ![]() Hence, in term of radiation heat transfer, q":
Hence, in term of radiation heat transfer, q":![]()
![]() of a real surface as the ratio of the thermal radiation emitted by a surface at a given temperature to that of a blackbody at the same temperature and for the same spectral and directional conditions.
of a real surface as the ratio of the thermal radiation emitted by a surface at a given temperature to that of a blackbody at the same temperature and for the same spectral and directional conditions.![]()
![]() The heat transfer rate by radiation for a non-blackbody, per unit area is defined as:
The heat transfer rate by radiation for a non-blackbody, per unit area is defined as:![]()

![]()

![]() If the total irradiation G (W/m2) represents the rate at which radiation is incident per unit area from all directions and at all wavelengths, it follows that:
If the total irradiation G (W/m2) represents the rate at which radiation is incident per unit area from all directions and at all wavelengths, it follows that:![]()
![]()
![]() and the remaining absorption and reflection can be treated as surface phenomenon. In other words, they are controlled by processes occurring within a fraction of a micrometer from the irradiated surface. It is therefore appropriate to say that the irradiation is absorbed and reflected by the surface, with the relative magnitudes of
and the remaining absorption and reflection can be treated as surface phenomenon. In other words, they are controlled by processes occurring within a fraction of a micrometer from the irradiated surface. It is therefore appropriate to say that the irradiation is absorbed and reflected by the surface, with the relative magnitudes of ![]() depending on the wavelength and the nature of the surface.
depending on the wavelength and the nature of the surface.![]() is defined as the rate at which radiation is emitted per unit area at all possible wavelengths and in all possible directions from a surface, per unit wavelength
is defined as the rate at which radiation is emitted per unit area at all possible wavelengths and in all possible directions from a surface, per unit wavelength ![]() and per unit surface area.
and per unit surface area. ![]() is defined as:
is defined as:![]()
![]()
![]() is a flux based on the actual surface area, where
is a flux based on the actual surface area, where ![]() is based on the projected area. In approximating a blackbody, the radiation is almost entirely absorbed by the cavity. Any radiation that exits the cavity is due to the surface temperature only.
is based on the projected area. In approximating a blackbody, the radiation is almost entirely absorbed by the cavity. Any radiation that exits the cavity is due to the surface temperature only.![]()
![]() (b for blackbody) represents the intensity of radiation emitted by a blackbody at temperature T, and wavelength
(b for blackbody) represents the intensity of radiation emitted by a blackbody at temperature T, and wavelength ![]() per unit wavelength interval, per unit time, per unit solid angle, per unit area. Also, h = 6.626 x 10-24 J•s and k = 1.3807 x 10-23 J•K-1 are the universal Planck and Boltzman constants, respectively; co = 2.9979 x 108 m/s is the speed of light in a vacuum, and T is the absolute temperature of the blackbody in Kelvins (K).
Due to the fact that deviations appeared between experimental results and the equation, Planck suggested in 1900 a refinement that better fit reality:
per unit wavelength interval, per unit time, per unit solid angle, per unit area. Also, h = 6.626 x 10-24 J•s and k = 1.3807 x 10-23 J•K-1 are the universal Planck and Boltzman constants, respectively; co = 2.9979 x 108 m/s is the speed of light in a vacuum, and T is the absolute temperature of the blackbody in Kelvins (K).
Due to the fact that deviations appeared between experimental results and the equation, Planck suggested in 1900 a refinement that better fit reality:![]()

![]() and
and ![]() Planck's distribution shows that as wavelength varies, emitted radiation varies continuously. As temperature increases, the total amount of energy emitted increases and the peak of the curve shifts to the left, or toward the shorter wavelengths. In considering the electromagnetic spectrum, it is apparent that bodies with very high temperatures emit energy in the visible spectrum as wavelength decreases. Figure 2-4 also shows that there is more energy difference per degree at shorter wavelengths.
Planck's distribution shows that as wavelength varies, emitted radiation varies continuously. As temperature increases, the total amount of energy emitted increases and the peak of the curve shifts to the left, or toward the shorter wavelengths. In considering the electromagnetic spectrum, it is apparent that bodies with very high temperatures emit energy in the visible spectrum as wavelength decreases. Figure 2-4 also shows that there is more energy difference per degree at shorter wavelengths. 
![]() and setting the result equal to zero:
and setting the result equal to zero:![]()
![]() • K. This is known as Wien's displacement law. The dashed line in Figure 2-4 defines this equation and locates the maximum radiation values for each temperature, at a specific wavelength. Notice that maximum radiance is associated with higher temperatures and lower wavelengths.
• K. This is known as Wien's displacement law. The dashed line in Figure 2-4 defines this equation and locates the maximum radiation values for each temperature, at a specific wavelength. Notice that maximum radiance is associated with higher temperatures and lower wavelengths.